3D Printing stronger materials five times faster
It’s allowed the aerospace, medical, automotive, manufacturing and many other industries to customize parts and prototypes in ways they never could before. It has drastically increased flexibility and cost effectiveness while reducing waste and production time. But many 3D-printed materials aren’t the strongest.
A team of chemists and materials scientists at Sandia National Laboratories hopes to change that.
They’ve developed a new printing process that prints stronger non-metallic materials in record time, five times faster than traditional 3D printing.
“It opens up a whole new world of what you can build and what 3D materials can be used for,” said Samuel Leguizamon, materials scientist.
He led the team that developed SWOMP, which stands for Selective Dual-Wavelength Olefin Metathesis 3D-Printing. As indicated by its name, it uses dual-wavelength light, unlike the traditional printing process.
How 3D printing works
Traditionally, vat 3D printing is accomplished by irradiating a vat of photosensitive liquid resin in a desired pattern.
As the resin is exposed to light from beneath the vat, the resin cures and hardens into a polymer layer. The cured polymer is then lifted, and a new pattern is projected beneath to cure subsequent layers.
One challenge: As the polymer cures, it adheres to the previous layer and to the bottom of the vat. After each layer, the cured polymer must be slowly peeled from the vat to prevent damage, significantly slowing down the 3D printing process.
Fellow creator Leah Appelhans said it’s kind of like baking cookies.
“After you bake the cookies, you have to let them cool,” she said. “If you were to try to peel the warm cookie off the cookie sheet, it’s squishy and it breaks apart. The same thing would happen with a 3D printer if you tried to quickly print each layer. Your work would get deformed.”
Leguizamon, Appelhans, former Sandia employee Jeff Foster and polymer scientist Alex Commisso came up with a way to cool the “cookies” quicker.
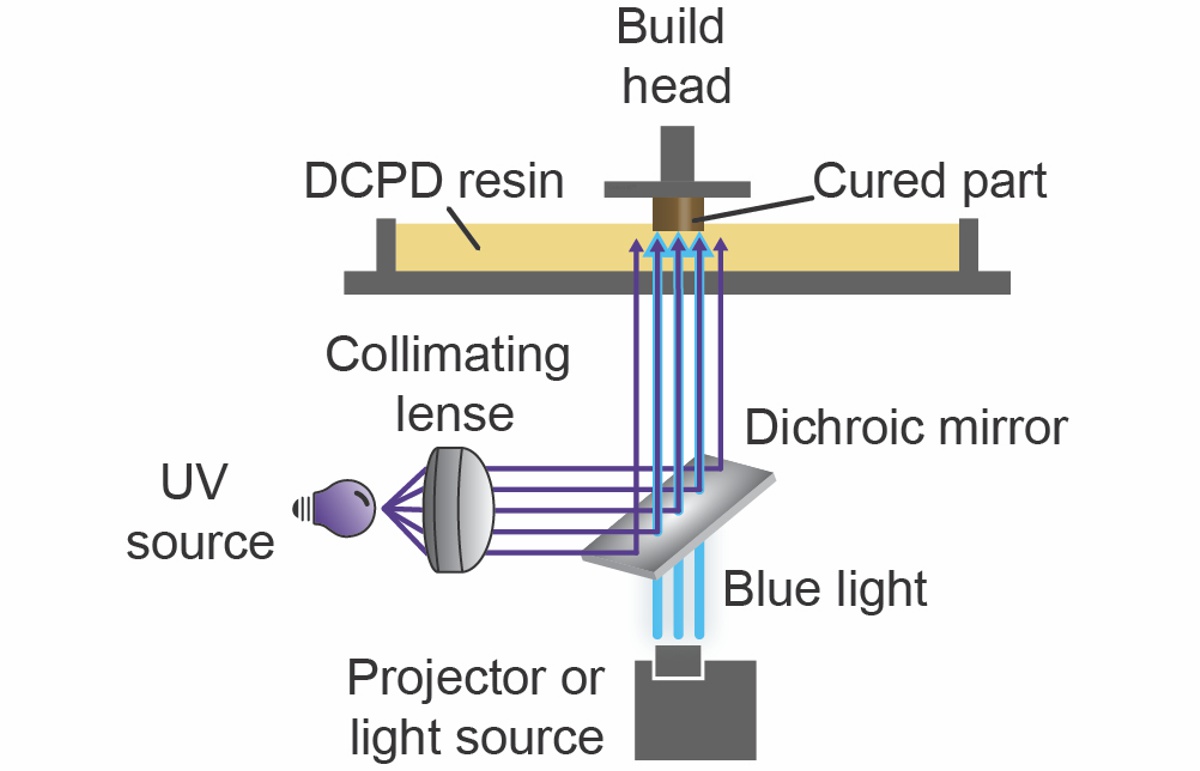
UV and blue light
The key is combining two lights. In this case, ultraviolet and blue light.
The team took inspiration from a technique known as continuous liquid interface printing along with a printing approach using dual-wavelength light for acrylic-based polymerizations.
With it, they created SWOMP.
“You are still printing layer by layer, but you are using a second wavelength of light to prevent polymerization at the bottom of the vat. So it doesn’t adhere to the bottom,” Leguizamon said. “That means you can lift the cured polymer part more quickly and speed up the printing process significantly.”

Making 3D materials stronger
But this new process isn’t just about efficiency. It’s about making 3D-printed materials stronger and more versatile. Most vat polymerization printed materials are acrylic-based, not the strongest material.
“It’s really hard to use these materials in things like aircraft and space and aerospace and automotive; they are very harsh environments,” Sandia licensing executive Bob Sleeper said.
This team turned to the material dicyclopentadiene, which is commonly used in the production of paints, varnishes and flame retardants for plastics. They were able to develop a way to polymerize it more rapidly with light so that it can be used more efficiently in 3D printing.
“We changed building blocks of the materials from acrylic-based to olefin-based,” Leguizamon said. “Which lets us print materials that are a lot tougher.”
“That is the beauty of what they are doing,” Sleeper said. “You have very high-quality plastic parts that are made very precisely by using some light in a very novel way.”
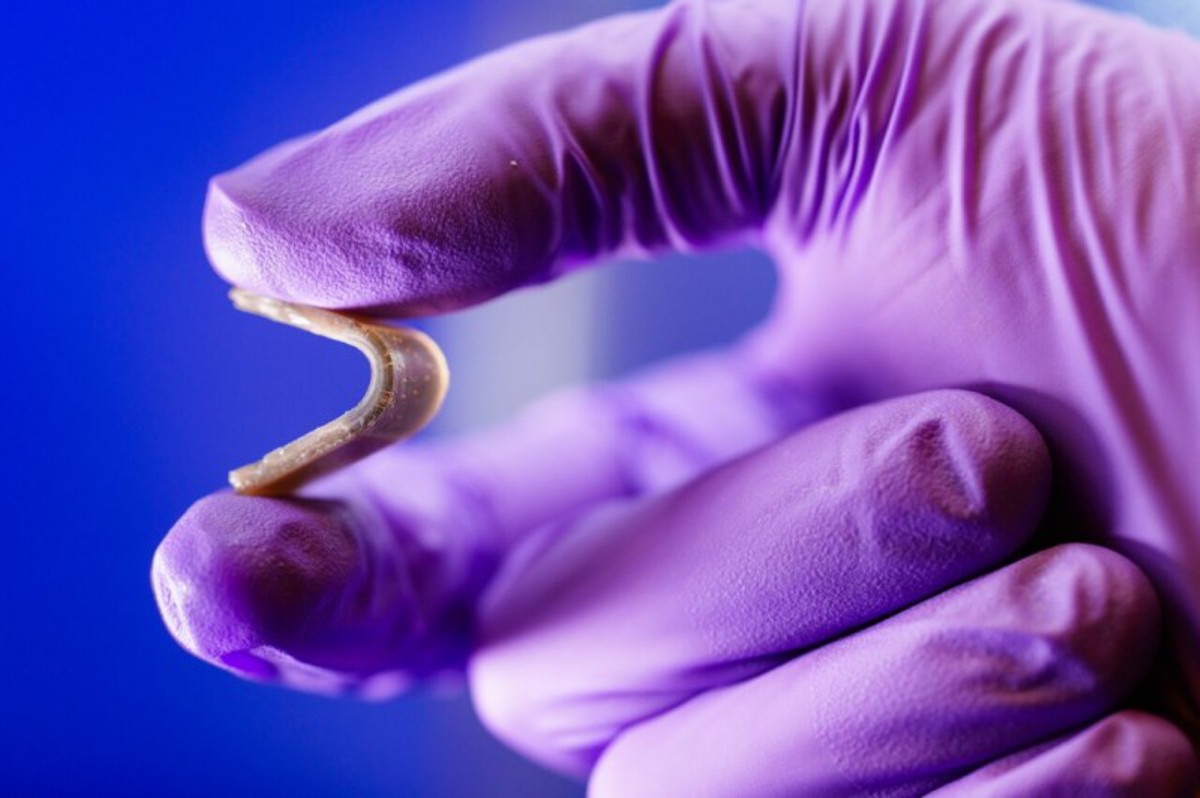
Opening a new world of 3D printing
This team hopes their new printing process will open the world of 3D printing.
While the project was initially funded through a rapid three-month Exploratory Express program, it’s now funded by a Sandia technology maturation program.
“What we are trying to do is build the toolbox of materials available,” Appelhans said. “We want designers, researchers, engineers to be able to select the type of material they want to use.”
One day, they hope to see these 3D-printed parts in rockets, engines, batteries and maybe even in fusion applications. Leguizamon said they’re already talking with researchers at Lawrence Livermore National Laboratory to explore applications.
“It turns out that monomers are already used in fusion components,” he said. “You don’t usually think of a polymer used in fusion, but it’s really cool and exciting potential.”
The team also sees a world where 3D printing can be done more easily in remote areas.
“We’re looking at locations where machinery and parts are not readily available; like in space, on the moon or in the Middle East at a U.S. military base,” Sleeper said. “You can bring with you some lightweight materials and make whatever you need on the spot.”
Leguizamon, who grew up in the small town of Wagener, South Carolina, is also thinking of applications that could help closer to home.
“I have horses,” he said. “I grew up in a rural area, my dad was a farrier, so I’m thinking of ways to make horseshoes for racehorses. They have to be impact-resistant, but by changing the material properties, stress can be better spread out and impact in the right space on the hoof. You could think of it as insoles for horses.”
The possibilities are endless.
“I think what attracted me to chemistry in the first place is the potential to make something that has never existed before,” Appelhans said. “The fun thing about 3D printing is that you apply that chemical knowledge to something that has a very concrete outcome. Something you can see and hold in your hands.”