Durable Fuel-Cells with novel proton-conductive membranes
Fuel cells are compact energy conversion units that utilize clean energy sources like hydrogen and convert them into electricity through a series of oxidation–reduction reactions.
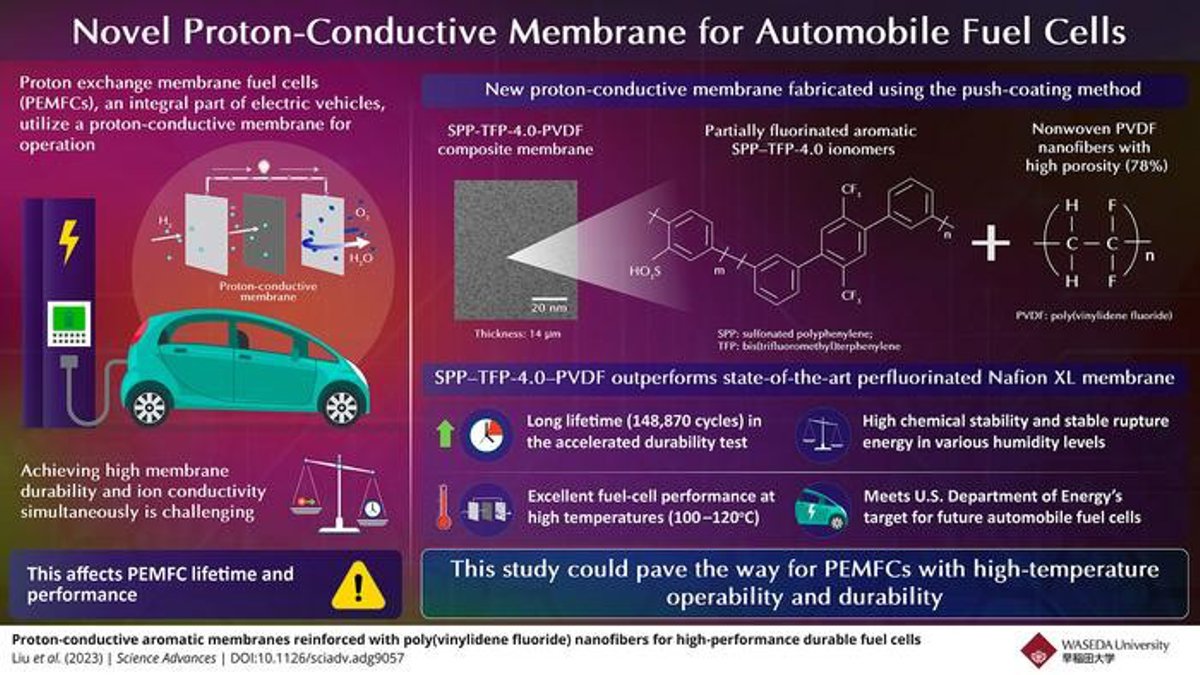
Specifically, proton exchange membrane fuel cells (PEMFCs), an integral part of electric vehicles, utilize proton-conductive membranes for operation. Unfortunately, these membranes suffer from a trade-off between high durability and high ion conductivity, affecting the lifetime and performance of PEMFCs.
To overcome this issue, scientists have synthesized chemically and physically modified perfluorosulfonic acid polymer membranes, such as Nafion HP, Nafion XL, and Gore-Select, which have proven to be much more durable than unmodified membranes conventionally employed in fuel-cell operations. Unfortunately, none of the existing proton-conductive membranes have fulfilled the highly challenging technical target—passing an accelerated durability test or a combined chemical and mechanical test—set by the U.S. Department of Energy (DOE) to facilitate their use in automobile fuel cells by 2025.
Against this background, a group of researchers from Japan, led by Professor Kenji Miyatake from Waseda University and the University of Yamanashi, has recently synthesized novel proton-conductive membranes for PEMFCs. Their work, published in the journal Science Advances, is co-authored by Dr. Liu Fanghua from Waseda University and the University of Yamanashi and Dr. Ick Soo Kim from Shinshu University.
The researchers synthesized proton-conductive membranes using a partially fluorinated aromatic ionomer (polymer material consisting of thermoplastic resins stabilized by ionic cross-linkages) called SPP–TFP-4.0 (SPP: sulfonated polyphenylene, TFP: bis(trifluoromethyl) terphenylene). They then utilized the push-coating method to reinforce the ionomer either with electrospun, nonwoven, and isotropic poly(vinylidene fluoride) (PVDF) nanofibers with high porosity (78%) or using porous expanded polytetrafluoroethylene (ePTFE). This resulted in composite membranes, SPP–TFP-4.0–PVDF and SPP–TFP-4.0–ePTFE, of thicknesses 14 and 16 µm, respectively.
The researchers performed a wide variety of tests on these proton-conductive membranes and demonstrated the one reinforced with PVDF to be superior. “It outperformed the state-of-the-art chemically stabilized and physically reinforced perfluorinated Nafion XL membrane in terms of fuel-cell operation and in situ chemical stability at a high temperature of 120oC and a low relative humidity of 30%,” highlights Miyatake.
The SPP–TFP-4.0–PVDF membrane demonstrated a long lifetime of 148,870 cycles or 703 hours—over seven times longer than that of the DOE target—in the accelerated durability test with frequent wet-dry cycling under open-circuit-voltage conditions. In addition, it exhibited high chemical stability with little degradation, stable rupture energy at various humidity levels, highly stable mechanical properties from zero to 60% relative humidity at 80oC, and excellent fuel-cell performance at high temperatures (100–120oC).
In effect, the proposed aromatic polymer-based reinforced proton-conductive membrane meets the U.S. DOE’s target for future automobile fuel cells, providing a lucrative alternative. Thus, this study could pave the way for PEMFCs with high-temperature operability and durability. “As a result, fuel cell-based electric vehicles may become more powerful and affordable. This would also contribute towards realizing a hydrogen-based, carbon-free society,” concludes an optimistic Miyatake.